Brain Protein Structure Offers Clues for Drug Design
Brain Protein Structure Offers Clues for Drug Design
Researchers have published the first highly detailed picture of how neurotensin, a molecule that plays an important role in the brain, interacts with its receptor. The achievement may help scientists design better drugs for certain disorders.
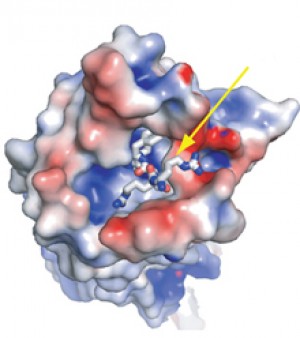
Neurotensin (see arrow) in the binding pocket of NTSR1. Image by Grisshammer and Tate labs, courtesy of Nature.
Neurotensin is a peptide of just 13 amino acids (too short to be called a protein). Because it acts on neurons, or nerve cells, it’s called a neuropeptide. When neurotensin binds its receptor, neurotensin receptor (NTSR1), it initiates a series of reactions. The neuropeptide plays many roles in the brain and also regulates digestive processes in the gut. Previous studies have shown that neurotensin may be involved in Parkinson’s disease, schizophrenia, temperature regulation, pain and cancer cell growth.
NTSR1 belongs to a class of membrane proteins called G-protein coupled receptors (GPCRs). The molecules that activate these receptors are called ligands. Previous X-ray crystallography studies showed that smaller ligands, such as adrenaline and retinal, bind in the middle of their respective GPCRs and well below the receptor’s surface.
A research team led by Dr. Reinhard Grisshammer of NIH’s National Institute of Neurological Disorders and Stroke (NINDS) set out to use X-ray crystallography to show what NTSR1 looks like in atomic detail when bound to neurotensin. Their study was supported by NINDS, NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and others. The report appeared online on October 10, 2012, in Nature.
In X-ray crystallography, scientists shoot X-rays at crystallized molecules to determine a molecule’s shape and structure. The X-rays change directions, or diffract, as they pass through the crystals. They then hit a detector where they form a pattern that is used to calculate the atomic structure of the molecule.
Forming well-diffracting neuropeptide-bound GPCR crystals is very difficult, and the researchers spent many years on the project. A group led by Dr. Christopher Tate at the MRC Laboratory of Molecular Biology in Cambridge, England, collaborated on the project. Tate’s lab used recombinant gene technology to create a stable version of NTSR1. Grisshammer’s lab employed the latest methods to crystallize the receptor bound to a short version of neurotensin.
The results suggest a novel binding mechanism for a neuropeptide to activate its target GPCR. Neurotensin binds to the outer part of its receptor, just at the receptor surface.
Atomic structures can guide the way scientists think about how proteins work. This finding may change the way scientists develop drugs targeting similar neuropeptide receptors.
“The knowledge of how the peptide binds to its receptor should help scientists design better drugs,” Grisshammer says. Nonetheless, he adds, more work will be needed to fully understand the detailed signaling mechanism of this GPCR.
###
* The above story is reprinted from materials provided by National Institutes of Health (NIH)
** The National Institutes of Health (NIH) , a part of the U.S. Department of Health and Human Services, is the nation’s medical research agency—making important discoveries that improve health and save lives. The National Institutes of Health is made up of 27 different components called Institutes and Centers. Each has its own specific research agenda. All but three of these components receive their funding directly from Congress, and administrate their own budgets.