New website eases clinical research trials — and tribulations
New website eases clinical research trials — and tribulations
The medical school has launched a new website that guides and supports biomedical researchers through the complex process of managing translational and human-subject research studies.
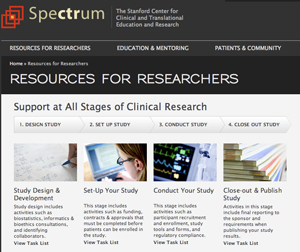 Developed by Spectrum, the Stanford Center for Clinical and Translational Education and Research, this online portal is part of an ongoing effort to streamline the infrastructure that supports this type of research across the university. It represents an important milestone in the Spectrum team’s mission to accelerate the flow of discoveries from university investigators to health practice. It was funded with the help of a Clinical and Translational Science Award from the National Institutes of Health.
Developed by Spectrum, the Stanford Center for Clinical and Translational Education and Research, this online portal is part of an ongoing effort to streamline the infrastructure that supports this type of research across the university. It represents an important milestone in the Spectrum team’s mission to accelerate the flow of discoveries from university investigators to health practice. It was funded with the help of a Clinical and Translational Science Award from the National Institutes of Health.
Ripping a page from physician-scribe Atul Gawande’s book, The Checklist Manifesto: How to Get Things Right, the website provides Stanford biomedical researchers with helpful to-do-lists for the four stages of running a clinical study — design, set-up, management and close-out. Easy-to-follow lists provide researchers with online links to essential clinical trial resources, such as funding websites and custom online budget workbooks, saving researchers time, trouble, and paperwork.
Within the Spectrum website, researchers also can access Study Navigator 2.0, a new release of Stanford’s clinical trial project tracker and collaboration tool. Since its launch in March, the development team has folded in user feedback to enhance research team workflow, especially in the area of documentation management.
“I find the document storage function of Study Navigator particularly useful,” said Karin Kushniruk, PhD, RN, a research nurse coordinator in maternal-fetal medicine. “This allows everyone on my study team to access, from one central online location, all pertinent documents, eliminating the risk of study staff inadvertently using outdated versions.”
Another popular feature of Study Navigator is the online calendaring function that enables researchers to instantly book appointments with experts in study design, biostatistics and informatics. Researchers can also request Lane Medical Library literature searches, community engagement advice and bioethics consultations from within Study Navigator.
“The Spectrum website will ultimately be the front door to all of Stanford’s clinical and translational resources, including a variety of training programs and pilot funding opportunities supported by Spectrum. It will help investigators focus on the research, not the administrative overhead of running a study,” said Harry Greenberg, MD, Spectrum’s director and the senior associate dean for research.
Future releases of Study Navigator will interface with other Stanford research management systems, such as eProtocol, SeRA and OnCore. Additionally, plans are under way to further integrate it with Clinical and Translational Research Unit services, formerly called the GCRC, increasing the overall availability of these support services across the university.
This new researcher resourceis available at http://spectrum.stanford.edu/
* Kris Newby is the communications manager for Spectrum, the Stanford Center for Clinical and Translational Education and Research.
![]() ** The above story is reprinted from materials provided by Stanford University School of Medicine
** The above story is reprinted from materials provided by Stanford University School of Medicine
________________________________________________________________
![]()