Learning How Support Cells Kill Nerve Cells in ALS
Learning How Support Cells Kill Nerve Cells in ALS
ALS, also known as Lou Gehrig’s disease, is marked by the death of muscle-controlling nerve cells—called motor neurons—in the spinal cord and some brain regions. As these neurons die, the body’s voluntary muscles weaken and waste away. People with ALS often die within 5 years of diagnosis. The only approved treatment, riluzole, extends life expectancy by just a few months.
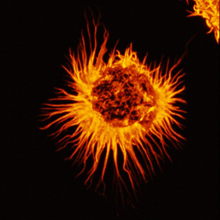
- Star-shaped astrocyte. Image by C. Merrifield and M. Duchen, Wellcome Images. All rights reserved by Wellcome Images.
Most people with ALS (about 90%) have sporadic disease, meaning the cause is unknown. The remaining 10% have a family history of ALS. A small portion of those with familial disease have mutations in the superoxide dismutase1 gene, SOD1. Although experts have suspected that the gene may hold clues to sporadic disease as well, the role of SOD1 in sporadic ALS has remained uncertain.
A research team led by Dr. Brian Kaspar of the Nationwide Children’s Research Institute in Ohio decided to take a closer look at the role of SOD1 mutations and astrocytes in both familial and sporadic ALS. Astrocytes are star-shaped supportive cells found primarily in the brain and spinal cord. The study was funded in part by NIH’s National Institute of Neurological Disorders and Stroke (NINDS).
As described in the August 10, 2011, advance online edition of Nature Biotechnology, the scientists studied astrocytes derived from recently deceased patients with either familial or sporadic ALS. When the team combined the human astrocytes with mouse motor neurons, they found evidence that the astrocytes secrete neuron-killing toxins. Earlier studies had found a comparable mechanism in mouse models of familial ALS. The new research showed that astrocytes derived from patients with familial and sporadic ALS were similarly toxic.
The researchers found that inflammatory responses may contribute to this toxicity. They analyzed 84 genes involved in inflammatory signaling. Up to 60% of the genes showed increased activity in ALS astrocytes compared to controls.
Further experiments showed that SOD1 plays a critical role in the toxicity. The scientists used a method called RNA interference to silence the SOD1 gene. This technique involves using small RNA fragments to block a gene from making proteins. When the researchers delivered SOD1-blocking RNAs to astrocytes affected by familial ALS, the astrocytes were no longer toxic to motor neurons. This method also suppressed toxicity in 4 of 6 astrocyte lines derived from people with sporadic ALS, supporting the idea that the SOD1 enzyme may also play a role in sporadic cases.
The new findings open the door for studying both sporadic and familial ALS in human cells, rather than relying on mouse models of familial ALS. “We still need to confront fundamental questions about what is happening to astrocytes and how they are killing motor neurons,” says Kaspar. “The ultimate goal is to identify therapies that will translate into helping humans.”
* The above story is reprinted from materials provided by National Institutes of Health (NIH)
** The National Institutes of Health (NIH) , a part of the U.S. Department of Health and Human Services, is the nation’s medical research agency—making important discoveries that improve health and save lives. The National Institutes of Health is made up of 27 different components called Institutes and Centers. Each has its own specific research agenda. All but three of these components receive their funding directly from Congress, and administrate their own budgets.