Research team identifies new mechanism with suspected role in cancer
Research team identifies new mechanism with suspected role in cancer
Researchers at Brown University and Rhode Island Hospital have identified a process in which prolactin receptors can be drawn together and begin working in pairs called dimers. Overexpression of prolactin receptors in patients has been linked to cancer.
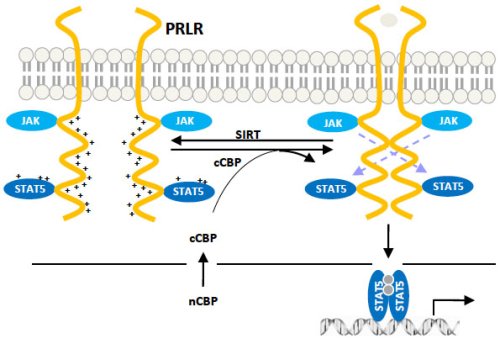
Working in pairs Under certain conditions, prolactin receptors (yellow in this schematic) can be joined to form dimers — working pairs — that researchers say could contribute to the incidence of cancer. Credit: Chin Lab/Brown University
PROVIDENCE, R.I. [Brown University] — If women had no prolactin receptors on cells in their mammary glands, they would not produce milk when they were nursing. Prolactin receptors are also found in other organs including the lung and the colon. The only problem is that these receptors are sort of like cellular wiring, and when the wrong conditions bring them together, the resulting short circuit can produce cancer.
In new research published online Oct. 18, 2010, in the Proceedings of the National Academy of Science, a team led by researcheres at Brown University and Rhode Island Hospital has identified a key chemical process by which cells with prolactin receptors can sometimes take that turn for the worse.
That key step is called “acetylation” — a chemical reaction inside the cell, triggered by the binding of the arrival of the prolactin hormone at the receptor. That process can draw prolactin receptors together into a structure called a “dimer.” Like a pair of chopsticks, this dimer structure is just right to pick up growth factors in the body that can lead to cancerous growth, said Y. Eugene Chin, associate professor of surgery (research) in the Warren Alpert Medical School of Brown University, based at Rhode Island Hospital.
“Our findings may provide an important clue about how to develop drugs to break down receptor dimers in breast cancer therapy,” said Chin, a senior author on the paper that also involved researchers from Zhejiang University School of Medicine in China and the University of Rochester in Rochester, N.Y.
Normally, a shared positive electrical charge and the resulting mutual repulsion keeps prolactin receptors from coming together. In their experiments, the team found that when prolactin binds to the receptors outside the cells, the acetylation neutralizes that charge on the receptors inside the cells, allowing the receptor molecules to come together, Chin said.
The more prolactin receptors a cell has, the more susceptible it is to this problem occurring, Chin said. Overexpression of prolactin receptors in patients has been linked to cancer in the past.
Chin, who has been investigating the molecular basis of cancer for years, said he is encouraged about uncovering this new step. He points to drugs, such as Herceptin, that target receptors to combat cancer.
“This will be extremely important for breast cancer and other cancer therapy by targeting receptors,” he said.
One possibility will be developing monoclonal antibodies to target the prolactin receptors directly, he said. But artificial compounds could also be developed to block the receptors from joining as dimers.
The research was funded by the National Institutes of Health.
In addition to Chin, other authors on the paper are Li Ma, Jinsong Gao, Yingjie Guan, Xiaoyan Shi, Hao Zhang, Marina Ayrapetov, and Shougang Zhuang, all of Brown and Rhode Island Hospital; Zhe Zhang of both Brown and Zhejiang University; Li Xu of Zhejiang; and Young-Min Hyun and Minsoo Kim of the University of Rochester.
* The above story is reprinted from materials provided by Brown University
** More information at Brown University (Providence, Rhode Island, USA)