A ‘Pacman Strategy’ to Boost the Immune System to Fight Cancer
A ‘Pacman Strategy’ to Boost the Immune System to Fight Cancer
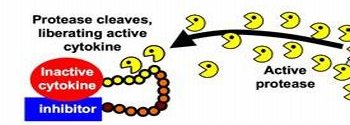
The key feature of the work is a new type of fusion molecule with three parts: a potent immune cell activator; a second molecule to keep that molecule quiescent until it’s needed; and a link between the two that gives scientists control over how the two interact.
The overall fusion molecule acts like a tiny anti-cancer grenade: The portion designed to arouse the immune system to attack cancer is inactive until it’s freed, an act that occurs when the link between it and its inhibitory counterpart is cleaved by specialized tumor proteins that chew up such molecules.
The work, led by graduate student John Puskas and Professor John Frelinger, Ph.D., was published online recently in the journal Immunology. Puskas, who is defending his doctoral thesis today, is first author of the paper.
In its experiments the team used Interleukin-2 or IL-2, a cytokine or chemical messenger that amplifies the effects of the immune system. IL-2 has been central to the burgeoning field known as cancer immunotherapy; it turns on T cells and natural killer cells that recognize and kill cancer cells. It’s approved by the U.S. Food and Drug Administration for the treatment of melanoma and kidney cancer, but it can have serious side effects, limiting its use in patients dramatically. That’s largely because it can harm healthy tissue when it’s active throughout the body.
“One reason we chose IL-2 is that it’s approved and used to treat patients today. If we’re able to reduce the toxicity associated with it, perhaps it could be used more broadly,” said Frelinger, professor of Microbiology and Immunology.
In experiments using the technology in the lab, the activity of IL-2 in the fusion protein was weak but became 10 to 50 times more biologically active after cleavage. Importantly, in experiments in mice with cancer, tumor growth was inhibited in mice where IL-2 was turned on using the technology compared to mice in which it was not. In many of the treated mice, tumor cells could not be detected after one week.
A key to the technology is the molecular link between IL-2 and its inhibitor. Puskas and Frelinger built that link out of a chain of amino acids – building blocks of proteins. Such chains are broken or cleaved constantly in the body by enzymes known as proteases. In these experiments, when the link is broken, IL-2 breaks free from its inhibitor and is suddenly available to activate other immune cells.
Puskas and Frelinger created links that are cleaved by molecules found much more commonly in cancer cells than other cells. For instance, in one set of experiments, they created a link that is broken only by prostate specific antigen, a protease that is found in prostate cancer cells. They also created links that are cleaved by proteases known as MMP2 and MMP9 – both examples of matrix metalloproteinases commonly overactive in many types of tumors.
The approach is designed to turn on the immune system powerfully right in the neighborhood of cancer cells, to destroy those cells, but to avoid a system-wide immune response that could cause severe side effects.
Frelinger points out that the new work is quite different from other experimental anti-cancer efforts that have involved fusion proteins. In other fusion protein approaches, the molecules are active throughout the body. In the new work, the cytokine is designed to be active only near tumor cells, an approach designed to reduce unwanted side effects.
“The beauty of this approach is that you can change any part of the molecule you want,” said Frelinger, who also has an appointment in the University’s James P. Wilmot Cancer Center. “If you want to target a specific type of cancer, you change the protease sequence to tailor it to particular types of tumors. If you want to change the part of the immune system activated, you change the cytokine.
“Our hope is that an approach like this might someday be coupled with other types of therapy, so that the body could initiate and maintain a vigorous immune response to kill tumors.”
Other authors besides Puskas and Frelinger include graduate students Denise Skrombolas and Abigail Sedlacek, and faculty members Edith Lord, Ph.D., and Mark Sullivan, Ph.D. The work was supported by the National Institutes of Allergy and Infectious Diseases as well as by Steven and Alison Krausz and F.C. Blodgett.
“John was very brave for taking on this project,” said Frelinger. “It really was something that hadn’t been attempted before. He did an outstanding job.”

Professor John Frelinger, Ph.D.,
* For Media Inquiries: Tom Rickey (585) 275-7954
** The above story is reprinted from materials provided by University of Rochester Medical Center