The Mechanism of Muscle Loss in Cancer
The Mechanism of Muscle Loss in Cancer
Factors released from tumors can block muscle repair, according to a new study. The finding partly explains why people with cancer often lose muscle. It also suggests a new avenue for treating the condition.
Cancer wasting, also called cancer cachexia, is marked by weakness and the progressive loss of body weight, fat, and muscle. The condition is responsible for 20-30% of cancer deaths and is currently untreatable. Knowing how tumors cause muscle loss could lead to life-saving treatments.
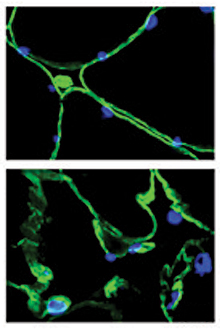
Cross-sections of muscle fibers from someone without cancer (top) and someone with pancreatic cancer (bottom). The damage from cancer is visible under the microscope. /Image by Guttridge lab, courtesy of JCI.
Lost muscle normally regenerates from a pool of ever-present stem cells. These cells remain in an immature state until muscle damage or loss causes them to commit to become (or differentiate into) muscle cells. The cells then fuse with surrounding muscle fibers to increase muscle mass. Defects in this process have been linked to various muscle-wasting diseases.
A team led by Dr. Denis Guttridge at Ohio State University set out to examine tumor-induced muscle injury and regeneration. They used staining techniques and biomarkers to track muscle loss and regeneration in tumor-bearing mice and pancreatic cancer patients. The work was funded in part by NIH’s National Cancer Institute (NCI) and National Center for Advancing Translational Sciences (NCATS). The results appeared online on October 1, 2013, in the Journal of Clinical Investigation.
In both mice and cancer patients, wasting was associated with muscle damage and activation of nearby stem cells. However, the stem cells became arrested in a semi-differentiated state and failed to fuse with the other muscle fibers. The patients weren’t able to replenish damaged muscle because their stem cells couldn’t fully differentiate into new muscle cells.
Next, the team tested whether the arrest of stem cell differentiation was caused by factors coming from the tumor. When arrested stem cells from wasting mice were removed from the tumor environment and placed in tumor-free culture, they were able to complete differentiation to muscle cells. When tumors were excised from wasting mice, the stem cells in the mice were able to complete their differentiation and fuse into muscle fibers. Consequently, the mice regained muscle mass.
The team searched for the factors responsible for this arrested differentiation. They found that overexpression of Pax7, which regulates muscle stem cell proliferation, impairs the stem cells’ differentiation to muscle cells in mice with cachexia. Further experiments showed that Pax7overexpression is controlled by NF-κB, a factor known to regulate formation of skeletal muscle. When mice with tumors received a treatment that interfered with production of Pax7, the mice showed a noticeable increase in muscle mass. Similarly, depleting NF-κB spared muscle atrophy in tumor-bearing mice.
“Our study showed that although muscle stem cells are activated during cachexia, factors released by the tumor block these cells from differentiating into muscle cells, which leaves them unable to repair cachectic muscle fibers,” Guttridge says. “By identifying agents that overcome the block and allow muscle stem cells to differentiate, it might be possible to restore muscle mass and enhance the quality of life of cancer patients with cachexia.”
By Katherine Wendelsdorf, Ph.D.
###
* The above story is reprinted from materials provided by National Institutes of Health (NIH)
** The National Institutes of Health (NIH) , a part of the U.S. Department of Health and Human Services, is the nation’s medical research agency—making important discoveries that improve health and save lives. The National Institutes of Health is made up of 27 different components called Institutes and Centers. Each has its own specific research agenda. All but three of these components receive their funding directly from Congress, and administrate their own budgets.