‘Genetic biopsy’ of human eggs might help pick the best for IVF
‘Genetic biopsy’ of human eggs might help pick the best for IVF
Researchers at Brown University and Women & Infants Hospital of Rhode Island have developed a way to extract information about gene expression from fertile human egg cells without hurting them. Expendable ‘polar bodies’ in the cells reflect much the same information as the eggs themselves, researchers have determined.
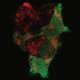
- Genetic information by proxy. Researchers found that analyzing genetic material in polar bodies can yield information about gene expression in the egg without disturbing the egg itself. Credit: Brown University
PROVIDENCE, R.I. [Brown University] — Given the stakes of in vitro fertilization, prospective parents and their doctors need the best information they can get about the eggs they will extract, attempt to fertilize, and implant. New research at Brown University and Women & Infants Hospital of Rhode Island has found a way to see which genes each egg cell is expressing without harming it. As researchers learn more about how those genes affect embryo development, the new technique could ultimately give parents and doctors a preview of which eggs are likely to make the most viable embryos.
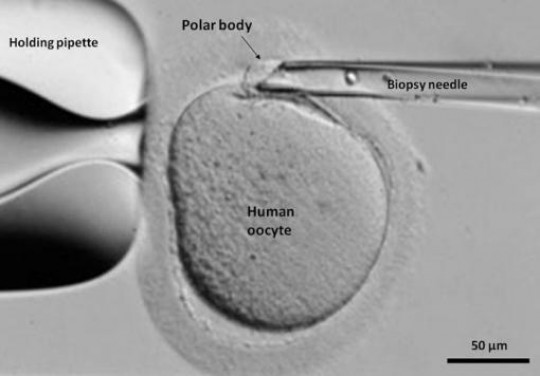
In the research, now in press in the Journal of Biological Chemistry, the team of physicians and biologists was able to sequence the transcribed genetic material, or mRNA, in egg cells and, in a scientific first, in smaller structures pinched off from them called “polar bodies.” By comparing the gene expression sequences in polar bodies and their host eggs, the researchers were able to determine that the polar bodies offer a faithful reflection of the eggs’ genetic activity.

- Sandra Carson. Polar bodies allow “a natural cytoplasmic biopsy,” providing genetic information without hurting the egg cell.
“We can now consider the polar body a natural cytoplasmic biopsy,” said study co-author Sandra Carson, professor obstetrics and gynecology at the Warren Alpert Medical School of Brown University and director of the Center for Reproduction and Infertility at Women & Infants Hospital.
Polar bodies are where egg cells dispense with the second copies of chromosomes that, as sex cells, they don’t need. But the polar bodies also capture a microcosm of the egg’s mRNA, the genetic material produced when genes have been transcribed and a cell is set to make proteins based on those genetic instructions.
Pairs of genes
Last year the team became the first to find mRNA in human polar bodies. Now they have transcribed it in 22 pairs of human eggs and their polar bodies, and confirmed that what is in the polar bodies is a good proxy for what is in the eggs.
Given how little mRNA is present in polar bodies, the task was not easy, said Gary Wessel, professor of biology, but through a combination of clever amplification and analysis techniques by lead author and graduate student Adrian Reich and second author Peter Klatsky, the team got it done.
“There’s no reason this should have worked, just because there was so little material,” Wessel said. “Single-cell sequencing is very challenging.”
To hedge their bets the team analyzed most of the samples in two pools of 10 cells each, for instance comparing the mRNA in 10 eggs with the mRNA in the 10 related polar bodies. But to their pleasant surprise, they were also able to sequence two individual eggs and their polar bodies directly.
What they found is that more than 14,000 genes can be expressed in the eggs. Of those, more than 90 percent of the genes detected in the polar bodies were also detected in the eggs and of the 700 most abundant genes found in the polar bodies, 460 were also among the most abundant in the eggs.

Gary Wessel. The team devised a way to get maximum information from a small amount of genetic material. “There’s no reason this should have worked.”
Toward clinical use
“It seems that the polar body does reflect what is in the egg,” Carson said. “Because the egg is the major driver of the first three days of human embryo development, what we find in the polar body may give us a clue into what is happening during that time.”
But Carson and Wessel acknowledged that more research will be required to create a clinically useful tool.
Finding which genes affect embryo viability is the next major step. With the new knowledge and techniques developed in their study, the researchers said, scientists could analyze the mRNA from polar bodies of eggs that are fertilized and track the progress of the resulting embryos. Once the key genes are known, they could create fast assays to look for those genes in polar bodies so that clinicians and patients could pick the best eggs. A sufficiently developed technology could also be used for choosing which eggs to bank for later use.
“We don’t quite have the answer of what those messages are doing exactly or necessarily the purpose of them in the cell function, but that’s to come,” Carson said. “Now we have the words, but not the sentences.”
The research was funded by seed grants from the Brown University Office of the Provost, the Center of Excellence in Women’s Health of Women & Infants Hospital, and Sigma-Aldrich, a research reagent supplier.
###
Sandra A. Carson
 Sandra A. Carson of Women and Infants Hospital, professor of obstetrics and gyneocology at the Warren Alpert Medical School of Brown University, has been elected vice president of the American Board of Obstetrics and Gynecology (ABOG).
Sandra A. Carson of Women and Infants Hospital, professor of obstetrics and gyneocology at the Warren Alpert Medical School of Brown University, has been elected vice president of the American Board of Obstetrics and Gynecology (ABOG).
ABOG is an independent, nonprofit organization that examines and certifies nearly 1,700 obstetrician-gynecologist and subspecialists in maternal-fetal medicine, reproductive endocrinology/infertility, and gynecologic oncology each year. Additionally, more than 18,000 physicians are examined annually for the purpose of maintenance of certification. ABOG also approves graduate medical education programs in the subspecialties of obstetrics and gynecology.
Carson is director of reproductive medicine and infertility at Women & Infants Hospital in Providence. She is a past president of the American Society for Reproductive Medicine and program chair of the Committee on the Scientific Program of the American College of Obstetricians and Gynecologists. Her research interests range from ectopic pregnancy and spontaneous abortion to in vitro fertilization and preimplantation genetic diagnosis.
###
Gary Wessel, PHD

Gary Wessel, PHD
Professor of Biology
Molecular Biology, Cell Biology, & Biochemistry
Phone: +1 401 863 1051
E-mail: Gary_Wessel@Brown.EDU
> Education
Undergraduate
University of Virginia, Charlottesville, Virginia
B.A. 1978; Biology and Environmental Sciences
Graduate
Duke University, Durham, North Carolina
Ph.D. 1986; Developmental Biology
Mentors: Drs. David R. McClay and Richard B. Marchase
Postgraduate
University of Texas M.D. Anderson Cancer Center,Houston
Department of Biochemistry and Molecular Biology
NIH Postdoctoral Fellow; May 1986 – April 1989
Sponsors: Drs. William H. Klein and William J. Lennarz
APPOINTMENTS
* University of Texas M.D. Anderson Cancer Center; Assistant Professor of Biochemistry and Molecular Biology; May 1989 – July 1990
* Brown University, Division of Biology and Medicine; Assistant Professor of Biology; July 1990 – July 1996
– Brown University, Division of Biology and Medicine; Associate Professor of Biology; July 1996 – 2001
– Brown University, Division of Biology and Medicine; Professor of Biology; June 2001 – present
– Marine Biological Laboratory, Woods Hole, MA; Senior Scientist (Brown); December 2005 – present
> Honors and Awards
– Fellow of the Marine Biological Laboratory, F.B. Bang Fellowship, Woods Hole, MA; 1991
– Basil O’Conner Starter Scholar Research Award, March of Dimes Birth Defects Foundation; 1992-1994
– Visiting Summer Scholar, Duke University Marine Laboratory; 1993, 1994, 1998-present
– National Institutes of Health Research Career Development Award, 1997-2002
– Visiting Scientist, Asamushi Marine Lab, University of Tohoku, March 1999, 2003
– Elizabeth H. LeDuc Award for Teaching Excellence in the Life Sciences, Brown University, 2003
> Funded Research
C. Research Projects Ongoing or Completed in the Last Three Years:
National Institutes of Health HD28152, 03/02 – 04/06
Title: Cortical Granule Regulation and Function
Principal Investigator: Gary M. Wessel
Goals: This project examines the regulation of the cortical granule protease, and the mechanisms of cortical granule translocation to the cell surface, followed by stimulus-dependent exocytosis.
NSF IOB-0620607, 08/06 – 09/09
Title: Primordial Germ Cell Determination in Basal Deuterostomes
Principal Investigator: Gary M. Wessel
Goals: This project examines the mechanism used by embryos to ascribe a fate of primordial germ cell to a small subset of its population.
National Institutes of Health HD28152, 04/08 – 03/12
Title: The Egg-to-Embryo Cell Surface Transition
Principal Investigator: Gary M. Wessel
Goals: This project examines the mechanisms of the cell surface changes that occur on the egg essential for initiating early embryonic development.
> Research
Fertilization and the block to polyspermy: Sperm-egg interactions result in a cascade of responses including exocytosis of cortical granules. This secretory event functions in the permanent block to polyspermy, important for fertilization, and in the formation of a protected environment for early embryonic development. We study the function of cortical granules in the modification of the egg cell surface and extracellular matrix using a molecular analysis of the protease and peroxidase activities, the structural proteins of the fertilization envelope, and the major protein of the extraembryonic matrix, hyalin.
Signaling Mechanisms in Oogenesis, Meiotic Maturation, and Fertilization: We examine the signal transduction pathways leading to normal cell function. These pathways target the release of free calcium into the cytoplasm, the generation of nitric oxide, and resumption of the cell cycle. We find a network of interacting molecular machines that include heterotrimeric G-proteins, and use yeast two-hydrid approaches to identify new components of each pathway.
Oocyte-Specific Transcriptional Mechanisms: Oocytes make several organelles specific to their cell type and transcribe a host of genes not active in any other cell. Some of these genes make the most abundant transcripts in the cell, which are then degraded selectively at maturation of the cell. Using GFP reporter gene constructs, we examine the gene’s cis-elements, and through use of bioinformatic approaches, have cloned and are testing specific transcription factors found greatly enriched in oocytes for their contribution to this cell’s phenotypic development.
> Affiliations
Trustee, Society for Developmental Biology; 1993-1996
Treasurer, Society for Developmental Biology; 1996-1999
NIH Research Career Development Award; 1997-2002
Marine Biological Laboratory, Woods Hole MA, Embryology course Faculty, 2003 – 2006
Editorial Boards:
Editor in Chief, Molecular Reproduction and Development, 2008 – present
Editor, Developmental Biology, 1998 – 2008
Executive Editor, Molecular Reproduction and Development, 2006 – 2008
Associate Editor, Molecular Reproduction and Development, 2000 – 2006
Member, Reproductive Biology and Endocrinology. 2002 – present
Member, Developmental Dynamics. 2002 – present
Member, BMC Developmental Biology, 2004 – present
Section Editor, MCB, BMC Biology Image Library, 2006 – present
* The above story is adapted from materials provided by Brown University
** More information at Brown University (Providence, Rhode Island, USA)