A New Way to Attack Pathogens …
A New Way to Attack Pathogens
RNA Recycling System Gone Awry Brings MRSA to a Halt
- Scientists have discovered a new way to attack dangerous pathogens
Scientists have discovered a new way to attack dangerous pathogens, marking a hopeful next step in the ever-escalating battle between man and microbe.
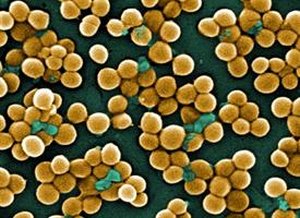
In a paper published online Feb. 10 in the journal PLoS Pathogens, scientists demonstrate that by stopping bacteria’s ability to degrade RNA – a “housekeeping” process crucial to their ability to thrive – scientists were able to stop methicillin-resistant Staphylococcus aureus or MRSA both in the laboratory and in infected mice.
The team, headed by a microbiologist at the University of Rochester Medical Center, is now developing closely related compounds designed to be much more potent than the one discussed in the paper.
The new approach shows promise against the most severe strains of MRSA as well as the toughest type of MRSA infection for antibiotics to infiltrate – bacteria enmeshed in biofilms.

Paul Dunman (center) with graduate student John Morrison and post-doctoral associate Christelle Roux
“This offers a whole new way to go on the offensive against some of the world’s most dangerous bugs,” said the leader of the group, Paul Dunman, Ph.D., associate professor of Microbiology and Immunology at the University of Rochester Medical Center and formerly of the University of Nebraska. “We’re hoping our research opens the door to an entirely new class of antibiotics.”
The team also includes scientists from the University of Nebraska, the University of Arkansas, Vanderbilt University, and the University of North Texas Health Science Center.
MRSA infections are among the most virulent infections known. The superbug causes nearly 500,000 hospitalizations and 19,000 deaths in the United States each year – more deaths than caused by AIDS. The bug can be acquired in the community or in hospitals.
MRSA and other dangerous microbes are so deadly largely because of their ability to adapt quickly to changing conditions. Bacteria’s knack for adaptation hinges on their ability to constantly churn out new molecules of RNA, which carry crucial messages that tell a cell what proteins to make and in what quantities.
The new research focuses on a critical cellular step that is part of the process, known as RNA degradation. Once an RNA molecule is no longer needed, the molecule is sliced and diced up, and its components are returned to the pool of available raw material that the cell taps again and again to construct other RNA molecules as needed.
“In bacteria, RNA degradation is crucial. The cells are replicating very quickly and responding to environmental changes very rapidly. In less than three minutes, a new RNA transcript is made, the protein is made, and then the RNA is degraded, and that material is made available to make other RNA molecules,” Dunman said.
Dunman’s team discovered that a molecule known as RnpA is central to the degradation process. After nailing down the activity of RnpA, the team tested more than 29,000 compounds in its search for one that inhibits its activity. The team found one, a small molecule called RNPA1000, that brings MRSA nearly to a standstill.
Throwing a monkey wrench into bacteria’s RNA recycling system might harm MRSA in a number of ways. One possibility is that since messenger RNA molecules are not destroyed like they should be, the bacteria are overcome by an array of confusing instructions that should have been turned off. Another possibility is that bacteria are unable to make essential new RNA molecules, since the supply of raw material is not available.
“We believe this basically makes the bacterial cell go haywire. The cell is producing proteins it no longer needs, and it can’t produce proteins that it does need,” said Dunman.
The team found that RNPA1000 is active against the predominant MRSA types circulating in the United States, vancomycin intermediate susceptible S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA). The compound also showed significant antimicrobial activity against a host of other bugs tested, including Staphylococcus epidermidis, antibiotic-resistant Streptococcus
pneumoniae, Streptococcus pyogenes, and vancomycin-resistant Enterococcus faecium.
Especially promising was its activity against MRSA biofilms, whose formation is central to the bacterium’s virulence in medical settings. Today’s antibiotics have a tough time breaking through MRSA biofilms on equipment like catheters, but RNPA1000 brought the bacteria to a halt even when they were ensconced within biofilms.
The agent does not affect other drugs used to treat MRSA infections, including vancomycin, daptomycin, or rifampicin; it does affect oxacillin, making it more potent. That find might make it possible to eventually combine an agent like RNPA1000 with other drugs that also target the infection.
The compound was also moderately effective in mice. In an experiment with infected mice, all of the untreated mice died from their infection, but half the mice survived when treated with a large dose of RNPA1000. The compound is also somewhat toxic to human cells at the largest doses. Those findings make it unlikely that RNPA1000 itself will end up as an antibiotic and spurred Dunman and colleagues to design safer, more potent alternatives.
“This is a great starting point,” said Dunman. “We’ve identified a compound that is very active against RnpA, and now we can use chemistry to try to increase its potency by hundreds of times, as well as make it less toxic to human cells. We’ve gotten a lead from the drug screen, and now we’re building a better molecule.”
Dunman is a leader in the development and use of array technology to study microbes and explore fresh approaches to the development of antibiotics. While microarrays are widely used by scientists to take what amounts to a snapshot of the activity of thousands of genes in a cell, Dunman adds a twist. His team employs the technology constantly to watch what happens to RNA molecules after they’ve been made, typically taking dozens of snapshots in the span of hours to get an ongoing, intimate look at RNA degradation.
The paper caps a six-year effort that began when the first author, Patrick Olson, was a high school student working as an intern in Dunman’s laboratory in Nebraska. Olson is now in graduate school at Washington University in St. Louis, working toward both his medical and doctoral degrees.
In addition to Dunman and Olson, other authors of the paper include post-doctoral associate Christelle Roux of the University of Rochester; Lisa Kuechenmeister, Kelsi Anderson, Tami Lewis, Oluwatoyin Asojo, and Khalid Sayood of the University of Nebraska; graduate student John Morrison of the University of Nebraska Medical Center, currently a visiting scientist in Dunman’s Rochester laboratory; Sonja Daily, Karen Beenken, and Mark Smeltzer of the University of Arkansas; Michelle Reniere and Eric Skaar of Vanderbilt University; and William Weiss, Mark Pulse, Phung Nguyen, and Jerry Simecka of the University of North Texas Health Science Center.
These researchers are among scientists at two dozen laboratories around the world with which Dunman works. He is also a founder and owner of Caddis Research LLC, which is developing antimicrobial agents that target bacteria that pose a threat to public health, and he is a consultant for Pfizer Research.
The project was funded by the National Institute of Allergy and Infectious Diseases, the American Heart Assn., and the Nebraska Research Initiative.

* The above story is reprinted from materials provided by University of Rochester Medical Center