Targeted Light Therapy Destroys Cancer Cells
Targeted Light Therapy Destroys Cancer Cells
Scientists have developed a noninvasive technique that uses light to selectively wipe out cancerous cells in mice without harming surrounding tissue. With further research, this novel method might eventually be used to treat tumors in humans.
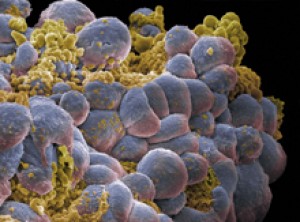
A cluster of breast cancer cells. (Image by Annie Cavanagh.
All rights reserved by Wellcome Images.)
The 3 major types of cancer therapy—surgery, radiation and chemotherapy—effectively destroy cancerous tissues, but tend to damage normal tissue as well. Researchers have long sought therapies that can zero in on tumor cells and leave neighboring healthy cells intact. One targeted technique, available for over a decade, is called monoclonal antibody (mAb) therapy. These antibodies destroy cancer cells by latching onto specific proteins on the cell surface. Researchers can also attach a deadly payload for mAbs to carry to target cells. Over 25 therapeutic mAbs have been approved by the US Food and Drug Administration to date. But current mAb therapies often require repeated doses at levels that can cause serious side effects.
To try a new approach, Dr. Hisataka Kobayashi and his colleagues at NIH’s National Cancer Institute (NCI) decided to couple mAbs with photosensitive dye molecules that respond to specific, harmless wavelengths of light. The unique method, called photoimmunotherapy, was described in the November 6, 2011, online edition of Nature Medicine.
After evaluating several light-sensitive molecules, the researchers settled on a fluorescent dye called IR700, which is activated by near infrared light. They chemically linked IR700 to 3 different mAbs. One antibody targets HER2, a cell-surface molecule that’s expressed at high levels by some breast cancer cells. Another binds to EGFR, a receptor molecule prevalent on some lung, pancreatic and colon cancer cells. The third mAb homes in on PSMA, a molecule overexpressed by prostate cancer cells.
The researchers found that the mAb-IR700 complexes effectively attached to cultured cancer cells. When exposed to near-infrared light, the targeted cells rapidly died. In contrast, cells that weren’t bound by the complexes were unharmed.
Mice with cancer were injected with the mAb-IR700 that targets EGFR. The animals showed dramatic tumor shrinkage after even a single dose of near-infrared light. The treatment appeared to be safe, with no signs of toxicity.
Other photosensitizing molecules have been used to treat cancer in the past. But these conventional, untargeted photosensitizers can damage both healthy and cancerous tissue. In addition, the type of light needed to activate these molecules can penetrate through less than 1 cm of tissue (about a third of an inch). The near-infrared light used to activate IR700 can penetrate tissue to a depth of several centimeters, more than an inch.
Low doses of mAb-IR700 can also help identify cancerous cells in tissues, because the complex emits a small amount of light. As treated tumors shrank, their fluorescence dimmed and eventually disappeared.
“The ability to join different mAbs to IR700 means that this technique might be used as a non-invasive guide to monitor the results of treatments,” says Kobayashi. “Although more testing will be needed, we believe this method has the potential to replace some surgical, radiation and chemotherapy treatments.”
###
* The above story is reprinted from materials provided by National Institutes of Health (NIH)
** The National Institutes of Health (NIH) , a part of the U.S. Department of Health and Human Services, is the nation’s medical research agency—making important discoveries that improve health and save lives. The National Institutes of Health is made up of 27 different components called Institutes and Centers. Each has its own specific research agenda. All but three of these components receive their funding directly from Congress, and administrate their own budgets.