Deadly virus reveals a potential weakness
Deadly virus reveals a potential weakness
A new study of the JC polyomavirus, a devastating pathogen that attacks brain cells in patients with compromised immune systems, has revealed how it binds to its targets, providing a basis for developing drugs to interrupt that process.
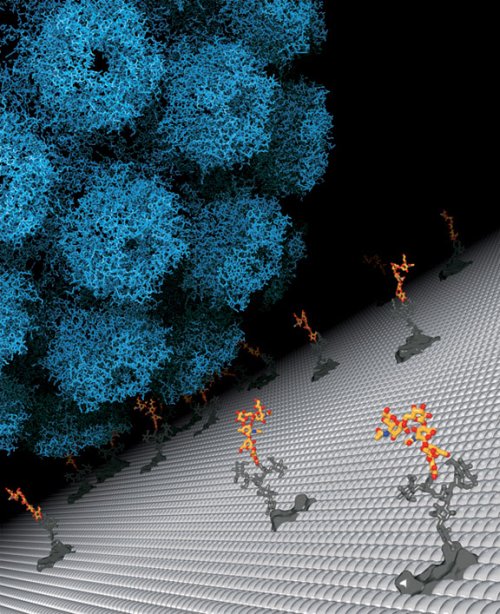
A fatal preference The virus approaches a cell, looking for a landing site. By figuring out which sugar molecule the JC polyomavirus prefers for binding, researchers have opened new possibilities for therapy and prevention. Credit: Ursula Neu/Tübingen University
PROVIDENCE, R.I. [Brown University] — The JC polyomavirus doesn’t strike very often, but it’s a mean bug that preys on people with weakened immune systems, including people with AIDS, and almost always kills them. Now an international team of scientists at Brown University, the University of Tübingen in Germany, and Imperial College in London has found a potential Achilles Heel and painted a target on it: The virus must bind to a very specific sugar molecule dangling from the side of the brain cells it attacks.
Like the rebel forces in the 1977 classic movie Star Wars, who smuggled and then analyzed detailed plans of the mighty Death Star fortress they had to destroy, the researchers painstakingly characterized the precise structure and biology of how the virus binds to host cells down to the atomic level. By exposing a specific target, their work, to be published Oct. 21, 2010, in the journal Cell Host & Microbe, sets the table for drug development to begin, said Walter Atwood, professor of molecular biology, cell biology, and biochemistry at Brown and a senior author of the new study.

Finding a vulnerability in the ‘Death Star’. Christian Nelson, Melissa Maginnis, and Walter Atwood helped figure out exactly where and how the JC polyomavirus binds to brain cells.
“The overall goal is to get these ‘plans’ and then design small molecules — drugs that will fit in this receptor, binding and preventing infection,” Atwood said.
Atwood noted that this paper also marks the first time anyone has fully determined the structure and binding functionality of a human polyomavirus. While the JC polyomavirus causes the brain-wasting disease known as PML, others in the “family” are implicated in ailments such as skin cancers.
Tübingen biochemist Thilo Stehle, also an author on the paper, said “solving” the virus was a hard-fought achievement.
“My group and the Atwood laboratory have worked for many years on defining attachment properties of human polyomaviruses,” he said. “In the end, our results provide a powerful platform for the development of new therapeutics that can now be developed based on the structural and functional data.”
Crystallizing an idea
When the virus floats toward a cell, it encounters a metaphorical cityscape of sugary molecules on its surface, said Brown postdoctoral researcher Melissa Maginnis, one of the paper’s two lead authors. The team wanted to know which one the virus chooses.
To finger a suspect, they turned to the lab of Ten Feizi, professor of medicine at Imperial College in London. After extensive screening experiments, Feizi and researcher Angelina Palma found that the virus strongly preferred to bind to sialic acid on the end of a molecule called LSTc.
From there, the Tübingen team, including Ursula Neu, another lead author, Luisa Stroh, and Stehle, crystallized the virus capsid protein VP1 with LSTc for imaging with x-rays at atomic resolution and showed exactly how the virus and LSTc bind.
Meanwhile the team at Brown, including Maginnis and postdoctoral researcher Christian Nelson, conducted experiments in which they sought further biological proof that binding with LSTc made the crucial difference between infection and health.
In one experiment, they pre-mixed the virus in some cases with LSTc and in others with the very similar molecule LSTb. Then they exposed each to glial cells. The virus pre-mixed with LSTc did not infect the cells, because they had already bound to LSTc in incubation (like a child who ruins an appetite by snacking before dinner). The virus that had been pre-exposed to LSTb, readily infected the glial cells. This told the researchers that the virus strongly “prefers” LSTc.
The team also created mutated versions of the virus’s binding protein to see whether any of the alterations would ruin is ability to infect cells. Different changes that made it more difficult for the virus to bind to LSTc also reduced the likelihood of infection to different degrees, showing that the binding to LSTc was what led to infection and also shedding light on the exact role each subpart plays.
The next step — finding a small-molecule drug that will cross the blood-brain barrier and bind to the virus so that it can’t bind with LSTc — is already getting underway in the Dartmouth College lab of Dale Mierke, who is a partner on the team’s grant from the National Institute of Neurological Disorders and Stroke.
For all the years they’ve put in, the researchers know they are still only in the middle of the fight, however.
“Drug development is a very long-term process,” Nelson said. “But the data in this paper provides the platform for rational drug design and opens the door to begin the process of screening compounds.”
* The above story is reprinted from materials provided by Brown University
** More information at Brown University (Providence, Rhode Island, USA)